Review of Evidence for Immune Evasion and Persistent Infection in Lyme Disease
Introduction
This Report focuses on the pathogenesis and pathophysiology of Lyme disease. In that location are other HHS TBDWG subcommittee reports that instead focus on clinical aspects of Lyme disease, and other tick-borne diseases, including problems related to the treatment of these diseases, that are posted on the HHS TBDWG website. Here nosotros summarize presentations by subcommittee members, as well every bit those of several other, invited investigators. It is recognized that there are many other important contributions past notable investigators in the area of pathogenesis and pathophysiology of Lyme disease and other tick-borne diseases that take not been included here, due to fourth dimension-limitations for the subcommittee.
Background
An understanding of the pathogenesis and pathophysiology of Lyme disease is primal to the ultimate care of patients with Lyme disease. To amend understand the various mechanisms underlying the infection acquired by Borrelia burgdorferi, the Pathogenesis and Pathophysiology of Lyme Disease Subcommittee was formed to review what is currently known about the pathogenesis and pathophysiology of Lyme disease, from its inception, but likewise specially near B. burgdorferi's ability to persist in the host. To that finish, the authors of this study were assembled to update our noesis about the infectious process, place the gaps that exist in our understanding of the process (Figure 1), and provide recommendations as to how to best approach solutions that could lead to a amend ways to manage patients with persistent Lyme illness.
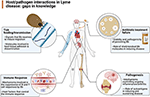
Figure 1. Overview of the gaps in knowledge defined past the working grouping equally areas in demand of farther inquiry (Created by Biorender.com).
It has been established that the major causative organism of Lyme disease, B. burgdorferi, can persist in a number of brute models and human instance studies post-obit infection and handling with a "standard" course of antibiotics (1–4). However, it is still unclear whether human being patients with ongoing symptoms associated with Lyme disease go along to accept an active infection following completion of what seems as appropriate antibody therapy. Thus, the extent to which unresolved infection, incomplete clearance of borrelial antigens, and/or autoimmunity contribute to persistent Lyme disease symptoms is unclear (five, 6).
To better sympathise the pathogenesis and pathophysiology of Lyme disease, the progression of B. burgdorferi from its reservoir in the Ixodes tick to transmission into the vertebrate host and to its localization and persistence in neural and other tissues are key steps toward finding means to resolve the infection. The following are descriptions of some of what is known nigh these various factors of the pathogenesis and pathophysiology of Lyme disease.
Transmission and Dissemination of B. burgdorferi in the Vertebrate Host
In the midgut of a molted, unfed tick, B. burgdorferi'south survival in a dormant state requires only a pocket-size corporeality of free energy, because little to no bacterial replication occurs (vii). Outer surface proteins (Osps) facilitate the pathogen's adhesion to midgut tissue. A tick's ingestion of claret provides B. burgdorferi with copious diet, resulting in rapid bacterial replication. In plow, B. burgdorferi stops producing tick-specific adhesins and starts producing OspC and other factors required for manual of the pathogen to vertebrates (viii). Subsequently initiation of a claret meal, the infected tick's midgut swells, and the junctions between midgut cells become thinner. Borrelia burgdorferi then penetrates those junctions and enters the tick's salivary glands and salivary ducts, thereby setting the stage for its manual to a vertebrate via tick seize with teeth. Upon injection into the vertebrate host, the bacteria adhere to tissues and replicate at the bite site (eight, nine). Dissemination of B. burgdorferi throughout the vertebrate host involves migration through tissues, also as transport via the bloodstream, resulting in a brief menstruation of bacteremia.
There are a number of questions meriting additional investigation, including processes occurring inside the tick, as well as the processes of initial entry and dissemination, such as the following:
• How does B. burgdorferi sense its location in the tick-mammal infectious wheel, so utilise that information to regulate production of its proteins?
• What are the signals that "tell" B. burgdorferi that a vector tick is feeding and that it is fourth dimension to transmit out of the tick?
• How does B. burgdorferi get into the tick'south salivary glands and salivary ducts?
• How does B. burgdorferi control production of host-specific proteins?
• When bacteria adhere to host tissues at the tick's seize with teeth site and and then replicate, to what kinds of tissues do they adhere? What types of proteins is B. burgdorferi making to facilitate adherence?
• Upon infection of a human, how does B. burgdorferi spread? Information technology is known to migrate through pare and other solid tissue, simply does it go through the lymphatic system or attach to nerve endings? Does it localize in sensory ganglia? What is the part of adhesins in dissemination throughout the vertebrate host? Are there particular host tissues that attract B. burgdorferi?
Gene Regulation of B. burgdorferi During Colonization, Dissemination, and Tissue-Specific Infection in Mice
Borrelia burgdorferi can sense whether it is located in a tick or mammal and adapt its response to environmental signals, such every bit temperature, pH, oxygen levels, carbon dioxide levels, nutrient availability, and reactive oxygen species (7). The rate of bacterial replication has effects on expression levels of numerous infection-associated genes and proteins. Carbon dioxide is important in determining the virulence of B. burgdorferi in mice. Borrelial oxidative stress regulator plays a pivotal role in establishing mammalian infection. B. burgdorferi can abound and survive without fe; genes generate an oxidative stress response that is involved in the send of manganese and other metals within B. burgdorferi-infected mice. The use of bioluminescent borrelia as a tool for studies in mice allows visualization of the kinetics of infection with dissimilar strains of the pathogen and enables real-fourth dimension evaluation of gene expression in the skin, center, and joints of a mammal infected with B. burgdorferi. Notably, localized infection with B. burgdorferi becomes more difficult to detect as the pathogen disseminates throughout the mouse. An important gap in knowledge is that it is all the same to be determined which genes are required for broadcasting of B. burgdorferi and its colonization of tissues during subsequently stages of infection (10).
Role of the Immune Organisation in Response to B. burgdorferi Infection
Borrelia burgdorferi establishes persistent and non-resolving infections in fully immunocompetent mice, strongly suggesting that the bacteria have developed multiple and likely complex immune evasion strategies (9, 11). Both innate and adaptive immune responses command B. burgdorferi in these hosts [reviewed in (12)]. These species rarely, and only transiently, develop clinical manifestations of disease, without an obvious correlation between the tissue-loads of B. burgdorferi and clinical manifestations, except in severely immunocompromised mice, for case those that lack T and B cells (SCID mice), or the ability to activate innate allowed effectors because of deletions in the toll-like receptor (TLR) 2 or TLR adaptor poly peptide MyD88 (13–15). MyD88-mediated innate immune responses appear to exist particularly critical during primeval stages during the establishment of infection (16). Immunoglobulin (Ig) G just non IgM antibodies control B. burgdorferi tissue loads, only cannot articulate the infection, even when the antibodies are able to passively protect from infection of a new host. IgG acts at to the lowest degree in office through complement-mediated opsonization of the leaner for subsequent update by macrophage and granulocytes. Information suggest that B. burgdorferi suppresses constructive innate and adaptive immunity (9, 11); therefore, the immune system is key to agreement persistence of Lyme illness.
B jail cell responses in these reservoir species are characterized by a lack of continued antibiotic analogousness maturation and the development of long-lived responses due to the rapid collapse of germinal centers. Borrelia burgdorferi infection appears to suppress the adaptive immune response, as indicated past the reduced immune response to flu vaccine in mice infected with B. burgdorferi (17). Ongoing work suggests that B. burgdorferi also prevents CD4 T cells from mounting an effective immune response to infection, potentially dysregulating effector immune responses in tissues and failing to suppress persistent infection of the host. Data were presented to back up the hypothesis that B. burgdorferi suppresses and subverts adaptive humoral and cellular immunity to itself and to other antigens. Identifying host immune targets of Borrelia-mediated immune suppression might result in the development of approaches that enhance host immunity to this pathogen in a mode similar to strategies that are currently being explored in anti-tumor immunity.
Notably, mice, as reservoir hosts, never articulate B. burgdorferi infection without antibiotic handling; humans and non-homo primates announced to harbor depression-level, persistent B. burgdorferi infection as well (18–xx). Persistence appears to be a function of active immune suppression and immune evasion tactics. An assay that was developed to detect antibody responses to 5 antigens of B. burgdorferi infection following antibiotic treatment (21) showed that almost rhesus macaques infected with B. burgdorferi generated responses to about of the antigens, but two showed no specific antibody responses to these antigens (22). In ane study in humans, patients who returned to health subsequently antibiotic handling generated the strongest antibody response (23), reflected by the percentage of plasmablasts that circulated in the blood (24), while those with persisting symptoms had weak responses to antigens or had an anti-oligopeptide permease A2 antibody titer that did not pass up. The reasons why some patients develop a skillful antibody response remain to be determined merely might be attributed to host immune factor differences or to differences in the infecting strains of B. burgdorferi.
Further studies of immune role in non-human primates previously vaccinated with B. burgdorferi found that IgM-producing cells were more frequent and persistent in B. burgdorferi-infected primates, results like to those observed in human patients with persistent Lyme disease equally well as in mice. Retentiveness B cells and plasmablasts were reduced in B. burgdorferi-infected, unvaccinated macaques compared with vaccinated macaques; whereas CD4 T-cell memory populations appeared like among groups, activation of T cells was somewhat dampened in the B. burgdorferi-infected primates. Areas for future research include determining how long B. burgdorferi-induced immune suppression lasts and the affect of persistent infection on effectiveness of vaccines.
Chemotaxis, Motion, and Immune Evasion as Key Factors in B. burgdorferi Spirochete Persistence
Most spirochetes apply flagellin proteins as "motors," with which they move back and forth. This movement can be tracked in real fourth dimension in mice with the use of multiphoton/confocal microscopy and fluorescently labeled B. burgdorferi. Ongoing imaging assay revealed that the number of spirochetes peaked around 7–x days afterwards infection (12). This peak was followed by a dramatic drop in spirochete numbers, where they persisted for the elapsing of the experiment. Spirochetes often tend to reside in the dermis. Of the various resident allowed response cells, Langerhans cells were not as effective as macrophages, or other dendritic cells or neutrophils in phagocytosing the bacteria, as spirochetes move up to 80 times faster than whatever of these immune response cells (12). Neutrophils responded the fastest, but after a sure signal, they stop responding, leaving a number of feasible spirochetes. There remains a gap in the understanding of the signals involved in this apparent suppression of neutrophil responses. Interleukin ten, the well-nigh well-characterized and immunosuppressive cytokine known, is induced early by B. burgdorferi to control the innate immune response (25). The innate allowed response is of import for controlling early on infection, independent of the presence of T and B cells. B. burgdorferi stimulates several design recognition receptors of the innate allowed response, inducing pro-inflammatory cytokines (26). Evasion of the innate immune response is achieved as well past multiple complement-binding proteins expressed past B. burgdorferi (27), dampening the initial response, also as IgG-mediated effort functions. Greater agreement is still needed regarding the different roles of the innate and adaptive arms of the immune system in regulating immunity to the spirochetes.
Function of CD47 and the Immune Response to B. burgdorferi
Up-regulation of CD47, a relatively conserved "marker of cocky," is a newly discovered mechanism of allowed evasion past B. burgdorferi. When CD47 binds to bespeak regulatory poly peptide alpha (SIRP-blastoff), there is an inhibition of phagocytosis of those cells, by macrophages. Anti-CD47 antibodies are currently under evaluation in clinical trials for cancer treatment (28, 29). It is hypothesized that B. burgdorferi (amongst other pathogens) tin can mimic CD47 and thus prevent macrophages from destroying Borrelia via phagocytosis (30). Imaging studies of the immune response to B. burgdorferi shows that macrophages can ship out a "lasso" that wraps around B. burgdorferi spirochetes and draws them into the macrophage, usually the first pace in the process of phagocytosis. In a few cases, the spirochetes reside in the macrophage but never appear to accomplish the lysosome, which is where bacterial destruction usually occurs. In donor sera, the addition of the SIRP-alpha binding domains of its receptor CV1G4 in vitro can consequence in increased phagocytosis, presumably by blocking serum-derived SIRP-blastoff to CD47-like molecules on the spirochete (31). To understand why the response is more efficient in some settings, the genetic sequences of CD47 and SIRP-blastoff were studied showing that SIRP-alpha is highly polymorphic (31). While a number of polymorphisms of CD47 do be, they are infrequent in humans. Evolutionarily, there has been long-term balancing choice, which ensures that proteins that are vital to the immune response are maintained with maximum diversity, perhaps because the pathogens see some types of SIRP-alpha every bit beneficial to them. By using mass spectrometry and CV1G4 as a bounden partner, a Borrelia poly peptide was identified equally a CD47-like anti-phagocytic signal. In the absence of this protein, macrophages were more effective in immigration cells. Whether B. burgdorferi tin survive by inhibiting phagolysosome fusion, as is the instance with a number of other known persistent pathogens (32), is currently unknown.
VlsE Poly peptide-Mediated Immune Evasion
VlsE is a surface-expressed protein able to undergo extensive antigenic variation (33–35). Its expression and ability to undergo antigenic variation is required for B. burgdorferi survival and persistence in the presence of a host humoral antibody response targeted against VlsE (36), but also against other surface proteins. A longstanding question has been how B. burgdorferi allowed escape is accomplished through sequence variation of this single lipoprotein can accomplish immune escape, despite the presence of a substantial number of additional antigens residing on the bacterial surface. A office for VlsE other than its antigenic variation, and thus constant evasion from the humoral antibody response, is not currently known to exist. Although other forms of immune evasion have been proposed, antigenic variation occurs fifty-fifty in antibody-scarce severe combined immunodeficient mice. Amongst the several models that accept been suggested, one scenario proposes that VlsE may act every bit a shield to obscure the epitopes of other surface antigens (37).
One case of this is the immunogenic Arp poly peptide of B. burgdorferi, which is responsible for joint inflammation during infection. Despite Arp eliciting a potent humoral response, antibodies fail to articulate the infection. Subsequent studies revealed that VlsE seems to forbid bounden of Arp-specific antibodies to the surface of B. burgdorferi, thereby providing a possible explanation for the failure of Arp antisera to clear the infection. However, other surface-expressed proteins of B. burgdorferi exercise not seem to be blocked past expression of VlsE, and Arp remains highly immunogenic. Thus, VlsE does non appear to exist a universal protector of all B. burgdorferi cell surface antigens. Therefore, other, as-of-yet-unknown mechanisms of immune evasion from antibody-mediated Borrelia clearance may exist.
Evidence That Persisting B. burgdorferi Are Metabolically Active and Induce Host Gene Expressions
Evidence now exists, from the results of experiments in both murine and non-homo primate models, that persisting B. burgdorferi can be metabolically active, expressing certain bacterial genes and inducing factor expression changes in the infected host, despite being non-culturable following antibiotic treatment (22, 37–39). In one model, the spirochetes localized to the dura mater of the brain, associated with large-calibration changes in gene expression of pro-inflammatory cytokines and chemokines (40, 41). Although there was no evidence of direct infection of the encephalon itself in this model, certain brain tissues expressed genes related to interferon signaling pathways. Gene expression of other brain functions—for example, glutamate receptors—have not yet been studied. These results, then, provide back up for the hypothesis that information technology is persisting infection that is the cause of persisting symptoms in patients with persistent Lyme illness.
One of the greatest challenges is to actually find means to intervene in the infectious process, especially if no specific markers tin can be found considering of low infectious load or if organisms are in locations other than blood, urine, or cerebrospinal fluid normally used for diagnosis of Lyme disease. Whether different antibiotic regimens can exist found to eliminate the persistent country is another claiming that it is hoped can be met with additional targeted inquiry.
Role of B. burgdorferi in the Pathogenesis and Persistence of Lyme Arthritis
Borrelia burgdorferi peptidoglycan, the principal component of the bacterial jail cell wall, has a unique composition and plays an important role in bacterial physiology and host immune responses. Borrelia burgdorferi lack the molecular machinery required for recycling of peptidoglycan during prison cell replication, and the leaner shed copious amounts of peptidoglycan fragments (42). These fragments are recognized by a host pathogen recognition receptor, NOD2, and cells stimulated with peptidoglycan fragments produce high levels of pro-inflammatory cytokines. Synovial fluid from some human patients with Lyme arthritis, many of whom had received 1–3 months of antibiotic therapy, had high levels of detectible peptidoglycan, as well as anti-peptidoglycan antibodies, despite a lack of whatever testify of ongoing infection after antibiotic therapy (42). Thus, it appears that B. burgdorferi peptidoglycan might be a persistent antigen in Lyme arthritis (12). Ongoing research is being conducted to determine whether B. burgdorferi peptidoglycan plays a role in the pathogenesis and pathophysiology of neuroborreliosis or of persistent Lyme disease other than previously treated Lyme arthritis.
Approximately 60% of untreated individuals with Lyme disease in the U.s.a. develop Lyme arthritis. Although most patients with Lyme arthritis respond favorably to 1–3 months of antibiotic therapy, ten–20% of patients take persistent arthritis afterwards treatment (43). A number of genetic and ecology factors contribute to persistent Lyme arthritis, such as infection past certain arthritogenic strains of B. burgdorferi, retained spirochetal antigens (for example, peptidoglycans), genetic take a chance factors, and evidence of prior joint trauma (43, 44). Equally in rheumatoid arthritis, the prototypical autoimmune articulation disease, Lyme arthritis is oft accompanied by autoimmune T- and B-cell responses to self-antigens (44). These unresolved inflammatory and autoimmune responses may contribute to ongoing arthritis, despite months of antibiotic therapy. Consistent with this hypothesis, nearly all patients with persistent Lyme arthritis experience resolution of arthritis when treated with immunosuppressive drugs, including non-steroidal anti-inflammatory drugs, corticosteroids, and other antirheumatic drugs, such as methotrexate or tumor necrosis factor-alpha inhibitors. Cellular analysis of the arthritic joint has shown that large numbers of IFN-gamma-positive lymphocytes are present in inflamed tissue and surrounding fluid (45). Synovial fibroblasts, the almost abundant jail cell blazon in synovial tissue, show evidence of immune activation and limited major histocompatibility complex (MHC) class Ii molecules and other allowed factors associated with inflammation and lymphocyte activation (44, 45).
Several self-peptides are immunogenic in Lyme disease patients, so there seems to exist a breakdown in immune tolerance to self during B. burgdorferi infection. Autoimmune B cell responses (but not T cell responses) can be detected early in infection in patients with erythema migrans, only these early autoimmune responses appear to be self-limiting and non-pathogenic. T cell autoimmunity accompanies B jail cell autoimmunity after in disease, such equally during Lyme arthritis. In late-stage affliction, Lyme-illness-associated autoantibodies correlate with clinical features of arthritis, suggesting that autoimmunity in Lyme affliction may become pathogenic over time. Lyme arthritis progresses from early invasion of synovial tissue to early on inflammatory responses to later inflammatory responses, and so to late tissue repair and wound healing (44, 45). The role of infection as an autoimmune trigger in Lyme disease is poorly understood, leading to the following questions:
• What are the mechanisms by which B. burgdorferi infection causes ongoing arthritic joint illness in a subset of patients?
• Are ongoing disease symptoms caused by the presence of Borrelia antigens (such as peptidoglycans) rather than active infection and, if and then, why are they not cleared from the host?
• Does Borrelia infection trigger autoimmune responses in infected individuals and are these autoimmune responses pathogenic in some patients?
Questions also remain regarding the role of immunosuppressive treatments vs. differing antibody treatment regimens for persistent Lyme arthritis, if peptidoglycan is an inflammatory agent and persists despite 1–3 months of antibiotic therapy. Patients who have persistent Lyme arthritis may correspond a different condition than do people with other Lyme disease syndromes.
Whereas, prompt treatment of early on Lyme disease, using antibiotics with differing mechanisms of action, is usually effective in prevention of persistence of B. burgdorferi and persistent Lyme disease, similar antibiotic treatments for persistent B. burgdorferi in animal models and in patients with persistent Lyme disease appear to be ineffective. The reasons for this difference are unclear, only may be due to a number of possible mechanisms:
• The leaner may be dormant or incapable of replication, however there may be the presence of residual antigens or the periodic release of antigens, to which the host responds to produce the symptoms associated with persistent Lyme disease.
• The bacteria may be entrenched in areas either inaccessible to certain classes of antibiotics (for case, poorly vascularized connective tissue, intracellular compartments), or higher doses of antibiotics are needed to attain levels that impede metabolic activity.
• The leaner may go antibiotic-tolerant, requiring repeated courses of antibiotic treatment, combinations of antibiotics, or periods of treatment alternating with periods of no treatment.
There are indications that sure handling regimens (for instance, tetracycline instead of doxycycline, the combination of a macrolide antibiotic and an alkalinizing agent) are effective in treating the persistent country if given over longer durations of fourth dimension rather than the usual ii–4-week periods. There is ongoing inquiry as well, some in the discovery phase, using novel compounds to treat persisting organisms. In that location is as well some indication that the intestinal microbiota may play an important role in the persistence or ability to eradicate persisting organisms.
Summary
The results of studies into the pathogenesis and pathophysiology of Lyme disease, with the focus on the persistent state of the causative organism, B. burgdorferi, have begun to elucidate the mechanisms underlying the process by which the persistent state occurs. Yet, important gaps exist into how the process develops, from the organism'southward beingness in the Ixodes tick, to its entry into the host, to its furnishings on the allowed system, to its distribution and ability to persist in certain tissues, to its ability to persist despite innate and other host immune system responses, and to its ability to persist despite certain antibody treatments. But there is reason for optimism that boosted enquiry into the pathogenetic and pathophysiologic mechanisms will lead to a improve understanding of the processes involved and ultimately to a better ways of preventing and treating patients with persistent Lyme affliction.
Author'south Notation
This report is a condensed version of the full written report that appears on the HHS TBDWG website, and has the permission of the HHS designated officer assigned to this working grouping.
Author Contributions
All authors contributed to the discussion and writing of the Written report.
Disclaimer
Data and opinions in this written report do not necessarily reflect the opinions of the Working Group, the U.S. Section of Health and Human being Services, or any other component of the Federal Regime. Readers should not consider the report or any part of it to be guidance or didactics regarding the diagnosis, care, or handling of tick-borne diseases or to supervene upon in whatever way existing guidance. All subcommittee members actively participated in the evolution of this study. Members voted to approve submission of the study to the Working Group and on the wording of each of the possible actions independent in the written report. The vote to submit the study indicates general agreement with the content of the document, merely it does not necessarily signal consummate agreement with each and every argument in the full report.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or fiscal relationships that could be construed as a potential disharmonize of involvement.
Acknowledgments
We acknowledge with dandy gratitude the technical back up of Jennifer Gillissen and her staff at Kauffman and Assembly, Inc.
References
1. Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. Spirochete antigens persist near cartilage later murine Lyme borreliosis therapy. J Clin Invest. (2012) 122:2652–lx. doi: ten.1172/JCI58813
PubMed Abstract | CrossRef Total Text | Google Scholar
two. Embers ME, Barthold SW, Borda JT, Bowers Fifty, Doyle L, Hodzic E, et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic handling of disseminated infection. PLoS One. (2012) 7:e29914. doi: 10.1371/journal.pone.0029914
PubMed Abstruse | CrossRef Full Text | Google Scholar
3. Sapi E, Kasliwala RS, Ismail H, Torres JP, Oldakowski M, Markland South, et al. The Long-term persistence of Borrelia burgdorferi antigens and DNA in the tissues of a patient with Lyme disease. Antibiotics (Basel). (2019) 8:183. doi: 10.3390/antibiotics8040183
PubMed Abstruse | CrossRef Full Text | Google Scholar
4. Strle F, Cheng Y, Cimperman J, Maraspin V, Lotric-Furlan S, Nelson JA, et al. Persistence of Borrelia burgdorferi sensu lato in resolved erythema migrans lesions. Clin Infect Dis. (1995) 21:380–ix. doi: 10.1093/clinids/21.2.380
PubMed Abstract | CrossRef Full Text | Google Scholar
5. Marques A. Chronic Lyme disease: a review. Infect Dis Clin North Am. (2008) 22:341–threescore, vii-viii. doi: 10.1016/j.idc.2007.12.011
CrossRef Full Text | Google Scholar
half dozen. Kullberg BJ, Vrijmoeth HD, van de Schoor F, Hovius JW. Lyme borreliosis: diagnosis and management. BMJ. (2020) 369:m1041. doi: 10.1136/bmj.m1041
CrossRef Full Text | Google Scholar
8. Jutras BL, Chenail AM, Stevenson B. Changes in bacterial growth rate govern expression of the Borrelia burgdorferi OspC and Erp infection-associated surface proteins. J Bacteriol. (2013) 195:757–64. doi: 10.1128/JB.01956-12
PubMed Abstract | CrossRef Full Text | Google Scholar
9. Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: agreement the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. (2012) x:87–99. doi: 10.1038/nrmicro2714
PubMed Abstract | CrossRef Full Text | Google Scholar
ten. Arnold WK, Savage CR, Brissette CA, Seshu J, Livny J, Stevenson B. RNA-Seq of Borrelia burgdorferi in multiple phases of growth reveals insights into the dynamics of gene expression, transcriptome architecture, and noncoding RNAs. PLoS I. (2016) 11:e0164165. doi: 10.1371/periodical.pone.0164165
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Tracy KE, Baumgarth North. Borrelia burgdorferi manipulates innate and adaptive immunity to establish persistence in rodent reservoir hosts. Front Immunol. (2017) eight:116. doi: x.3389/fimmu.2017.00116
PubMed Abstract | CrossRef Total Text | Google Scholar
xiii. Wooten RM, Ma Y, Yoder RA, Chocolate-brown JP, Weis JH, Zachary JF, et al. Cost-similar receptor ii plays a pivotal role in host defense and inflammatory response to Borrelia burgdorferi. Vector Borne Zoonotic Dis. (2002) 2:275–8. doi: x.1089/153036602321653860
PubMed Abstruse | CrossRef Full Text | Google Scholar
14. Bolz DD, Sundsbak RS, Ma Y, Akira South, Kirschning CJ, Zachary JF, et al. MyD88 plays a unique role in host defense only not arthritis evolution in Lyme disease. J Immunol. (2004) 173:2003–10. doi: 10.4049/jimmunol.173.3.2003
PubMed Abstract | CrossRef Full Text | Google Scholar
15. Behera AK, Hildebrand E, Bronson RT, Perides G, Uematsu S, Akira S, et al. MyD88 deficiency results in tissue-specific changes in cytokine induction and inflammation in interleukin-18-independent mice infected with Borrelia burgdorferi. Infect Immun. (2006) 74:1462–70. doi: 10.1128/IAI.74.iii.1462-1470.2006
PubMed Abstract | CrossRef Full Text | Google Scholar
16. Troy EB, Lin T, Gao L, Lazinski DW, Camilli A, Norris SJ, et al. Understanding barriers to Borrelia burgdorferi broadcasting during infection using massively parallel sequencing. Infect Immun. (2013) 81:2347–57. doi: 10.1128/IAI.00266-13
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Elsner RA, Hastey CJ, Olsen KJ, Baumgarth N. Suppression of long-lived humoral amnesty following Borrelia burgdorferi infection. PLoS Pathog. (2015) 11:e1004976. doi: 10.1371/journal.ppat.1004976
PubMed Abstruse | CrossRef Full Text | Google Scholar
eighteen. Crossland NA, Alvarez 10, Embers ME. Late disseminated Lyme disease: associated pathology and spirochete persistence posttreatment in rhesus macaques. Am J Pathol. (2018) 188:672–82. doi: x.1016/j.ajpath.2017.11.005
PubMed Abstract | CrossRef Full Text | Google Scholar
19. Coulter P, Lema C, Flayhart D, Linhardt Equally, Aucott JN, Auwaerter PG, et al. Two-yr evaluation of Borrelia burgdorferi culture and supplemental tests for definitive diagnosis of Lyme illness. J Clin Microbiol. (2005) 43:5080–4. doi: 10.1128/JCM.43.10.5080-5084.2005
PubMed Abstract | CrossRef Full Text | Google Scholar
20. Pachner AR, Zhang WF, Schaefer H, Schaefer S, O'Neill T. Detection of active infection in nonhuman primates with Lyme neuroborreliosis: comparison of PCR, culture, and a bioassay. J Clin Microbiol. (1998) 36:3243–7. doi: ten.1128/JCM.36.eleven.3243-3247.1998
PubMed Abstruse | CrossRef Total Text | Google Scholar
21. Embers ME, Hasenkampf NR, Barnes MB, Didier ES, Philipp MT, Tardo AC. Five-antigen fluorescent bead-based assay for diagnosis of Lyme disease. Clin Vaccine Immunol. (2016) 23:294–303. doi: 10.1128/CVI.00685-xv
PubMed Abstract | CrossRef Total Text | Google Scholar
22. Embers ME, Hasenkampf NR, Jacobs MB, Tardo AC, Doyle-Meyers LA, Philipp MT, et al. Variable manifestations, various seroreactivity and mail service-treatment persistence in non-human primates exposed to Borrelia burgdorferi by tick feeding. PLoS Ane. (2017) 12:e0189071. doi: x.1371/journal.pone.0189071
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Aucott JN, Crowder LA, Kortte KB. Development of a foundation for a case definition of mail service-handling Lyme disease syndrome. Int J Infect Dis. (2013) 17:e443–9. doi: x.1016/j.ijid.2013.01.008
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Blum LK, Adamska JZ, Martin DS, Rebman AW, Elliott SE, Cao RRL, et al. Robust B cell responses predict rapid resolution of Lyme disease. Forepart Immunol. (2018) 9:1634. doi: 10.3389/fimmu.2018.01634
PubMed Abstruse | CrossRef Full Text | Google Scholar
25. Brown JP, Zachary JF, Teuscher C, Weis JJ, Wooten RM. Dual function of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense force. Infect Immun. (1999) 67:5142–50. doi: 10.1128/IAI.67.10.5142-5150.1999
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Oosting M, Buffen K, van der Meer JW, Netea MG, Joosten LA. Innate immunity networks during infection with Borrelia burgdorferi. Crit Rev Microbiol. (2016) 42:233–44. doi: 10.3109/1040841X.2014.929563
PubMed Abstract | CrossRef Full Text | Google Scholar
29. Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human being solid tumors. Proc Natl Acad Sci U S A. (2012) 109:6662–7. doi: 10.1073/pnas.1121623109
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Tal MC, Torrez Dulgeroff LB, Myers L, Cham LB, Mayer-Hairdresser KD, Bohrer AC, et al. Upregulation of CD47 Is a host checkpoint response to pathogen recognition. mBio. (2020) 11:mBio.e-1293-xx. doi: 10.1128/mBio.01293-20
PubMed Abstruse | CrossRef Total Text | Google Scholar
31. Weiskopf Thousand, Ring AM, Ho CC, Volkmer JP, Levin AM, Volkmer AK, et al. Engineered SIRPalpha variants every bit immunotherapeutic adjuvants to anticancer antibodies. Science. (2013) 341:88–91. doi: 10.1126/scientific discipline.1238856
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Bankhead T, Chaconas G. The role of VlsE antigenic variation in the Lyme disease spirochete: persistence through a mechanism that differs from other pathogens. Mol Microbiol. (2007) 65:1547–58. doi: 10.1111/j.1365-2958.2007.05895.x
PubMed Abstruse | CrossRef Full Text | Google Scholar
34. Zhang JR, Norris SJ. Genetic variation of the Borrelia burgdorferi cistron vlsE involves cassette-specific, segmental cistron conversion. Infect Immun. (1998) 66:3698–704. doi: 10.1128/IAI.66.eight.3698-3704.1998
PubMed Abstract | CrossRef Full Text | Google Scholar
36. Bankhead T. Function of the VlsE lipoprotein in immune abstention by the lyme disease spirochete Borrelia burgdorferi. For Immunopathol Dis Therap. (2016) 7:191–204. doi: 10.1615/ForumImmunDisTher.2017019625
PubMed Abstract | CrossRef Total Text | Google Scholar
37. Greenmyer JR, Gaultney RA, Brissette CA, Watt JA. Main human microglia are phagocytically active and answer to Borrelia burgdorferi with upregulation of chemokines and cytokines. Front Microbiol. (2018) ix:811. doi: x.3389/fmicb.2018.00811
PubMed Abstract | CrossRef Total Text | Google Scholar
38. Caskey JR, Hasenkampf NR, Martin DS, Chouljenko VN, Subramanian R, Cheslock MA, et al. The functional and molecular furnishings of doxycycline treatment on Borrelia burgdorferi phenotype. Front Microbiol. (2019) x:690. doi: ten.3389/fmicb.2019.00690
PubMed Abstract | CrossRef Full Text | Google Scholar
39. Hodzic E, Imai D, Feng S, Barthold SW. Resurgence of persisting not-cultivable Borrelia burgdorferi following antibiotic handling in mice. PLoS Ane. (2014) 9:e86907. doi: ten.1371/journal.pone.0086907
PubMed Abstruse | CrossRef Full Text | Google Scholar
40. Casselli T, Divan A, Vomhof-DeKrey EE, Tourand Y, Pecoraro HL, Brissette CA. A murine model of Lyme disease demonstrates that Borrelia burgdorferi colonizes the dura mater and induces inflammation in the key nervous system. PLoS Pathog. (2021) 17:e1009256. doi: 10.1371/journal.ppat.1009256
PubMed Abstract | CrossRef Total Text | Google Scholar
41. Divan A, Casselli T, Narayanan SA, Mukherjee Due south, Zawieja DC, Watt JA, et al. Borrelia burgdorferi attach to blood vessels in the dura mater and are associated with increased meningeal T cells during murine disseminated borreliosis. PLoS ONE. (2018) thirteen:e0196893. doi: 10.1371/journal.pone.0196893
PubMed Abstruse | CrossRef Total Text | Google Scholar
42. Jutras BL, Lochhead RB, Kloos ZA, Biboy J, Strle K, Booth CJ, et al. Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis. Proc Natl Acad Sci U S A. (2019) 116:13498–507. doi: ten.1073/pnas.1904170116
PubMed Abstruse | CrossRef Full Text | Google Scholar
43. Arvikar SL, Steere Air-conditioning. Diagnosis and handling of Lyme arthritis. Infect Dis Clin North Am. (2015) 29:269–80. doi: 10.1016/j.idc.2015.02.004
CrossRef Full Text | Google Scholar
44. Lochhead RB, Arvikar SL, Aversa JM, Sadreyev RI, Strle Yard, Steere AC. Robust interferon signature and suppressed tissue repair factor expression in synovial tissue from patients with postinfectious, Borrelia burgdorferi-induced Lyme arthritis. Cell Microbiol. (2019) 21:e12954. doi: x.1111/cmi.12954
PubMed Abstract | CrossRef Full Text | Google Scholar
45. Lochhead RB, Ordonez D, Arvikar SL, Aversa JM, Oh LS, Heyworth B, et al. Interferon-gamma production in Lyme arthritis synovial tissue promotes differentiation of fibroblast-like synoviocytes into allowed effector cells. Jail cell Microbiol. (2019) 21:e12992. doi: 10.1111/cmi.12992
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fmed.2021.643235/full
0 Response to "Review of Evidence for Immune Evasion and Persistent Infection in Lyme Disease"
Post a Comment